Abstract
Introduction:
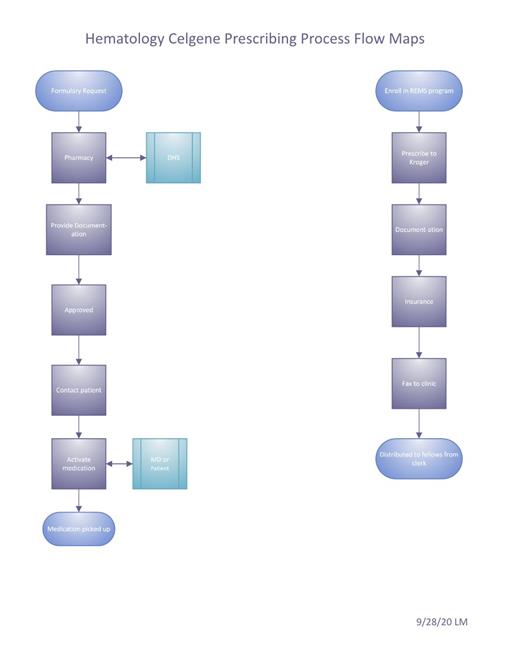
Lenalidomide is an immunomodulatory agent used primarily in the management of multiple myeloma and non-Hodgkin's lymphomas. Owing to the risks of birth defects and fetal death, lenalidomide is only available under a restricted program through Celgene called Revlimid Risk Evaluation and Mitigation Strategy (REMS). The Revlimid REMS program includes various requirements for patients and providers aimed to avoid embryo-fetal exposure from lenalidomide. Prescribers and patients are required to complete periodic mandatory surveys attesting that the patient is aware of the risks. Once the surveys are completed, a unique and time-limited authorization number is generated by Celgene, which must be included on the prescription. Each prescription is restricted to a 4-week supply with no automatic refills and must be sent to certified specialty pharmacy. In addition, the patient may require insurance authorization or may need to enroll in financial assistance program. Given this multi-step process, patients from our safety net hospital, LAC+USC Medical Center, experience delays in receiving their lenalidomide prescriptions. Such delays may lead to interruptions in cancer treatment and additional clinic visits. Our aim was to assess the effectiveness of the following interventions: creation of a standardized process flowchart, training of clinic staff, and additional patient support from Celgene to reduce delays in the dispensing of lenalidomide prescriptions.
Methods:
This is a retrospective study of patients prescribed lenalidomide through the hematology clinic at LAC+USC Medical Center, Los Angeles, CA from June 1, 2020 to December 31, 2020. Patients were identified through the Celgene REMS database. The electronic medical record was reviewed for: patient demographics, insurance, and specialty pharmacy dispensing of lenalidomide. Each prescription was reviewed for the authorization number, days from prescription submission to dispensing, and days between each prescription dispensing. A delay was defined as > 2 weeks from the time from prescription submission to pharmacy dispensing. The medical chart was reviewed to identify the reason for the delay.
A standardized process was created between July 2020 and August 2020. This included the creation of a workflow flowchart and training of the clinic staff. Additionally, a patient access specialist from Celgene was assigned to support patients and providers through the multi-step process for each prescription. The percentage of pre-intervention delays (before Sept 2020) and post-intervention delays (after Sept 2020) was compared.
Results:
A total of 196 lenalidomide prescriptions were reviewed. Prior to the intervention, the median time from prescription sent to date of dispensing was 3 days (range: 0-27 days), with a mean time of 5.9 days. 14 of 128 prescriptions (10.9%) had a delay of > 2 weeks. Causes for delay included: awaiting completion of patient survey, insurance issues (need for prior authorization, insurance changes), clinic visit missed or not in correct timeframe to submit new prescription, hospitalization, and medication hold due to toxicity.
Following the intervention, 3 of 68 prescriptions (4.4%) were delayed. Median time from prescription sent to date dispensed was 2.5 days (range: 0-29 days) with a mean time of 4.2 days. One prescription was not sent to the correct specialty pharmacy and one was on hold in setting of disease progression.
Conclusion:
Given the multi-step process, on-time dispensing of the specialty drug lenalidomide is a challenge at our safety net hospital. We identified several delays in the dispensing of lenalidomide prescriptions, including the timeliness of patient survey completion, drug coverage/insurance issues, and coordination of clinic visits with the time that the patient was due for the refill. Formalizing the workflow, training the clinic staff, and having a Celgene patient support specialist led to an improvement in prescription dispensing delays. With the continual addition of specialty medications into hematology/oncology clinics, establishing a standardized workflow with engagement of the clinic staff and specialty pharmacies/drug companies may help reduce delays in the dispensing of specialty drugs.
Piatek: Rigel: Consultancy, Research Funding; Alexion: Consultancy, Research Funding; Apellis: Research Funding; Dova: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal